Xinhua News Agency, Tianjin, February 16th Title: Tianjin's biopharmaceutical industry accelerates its move towards a 100 billion-level cluster
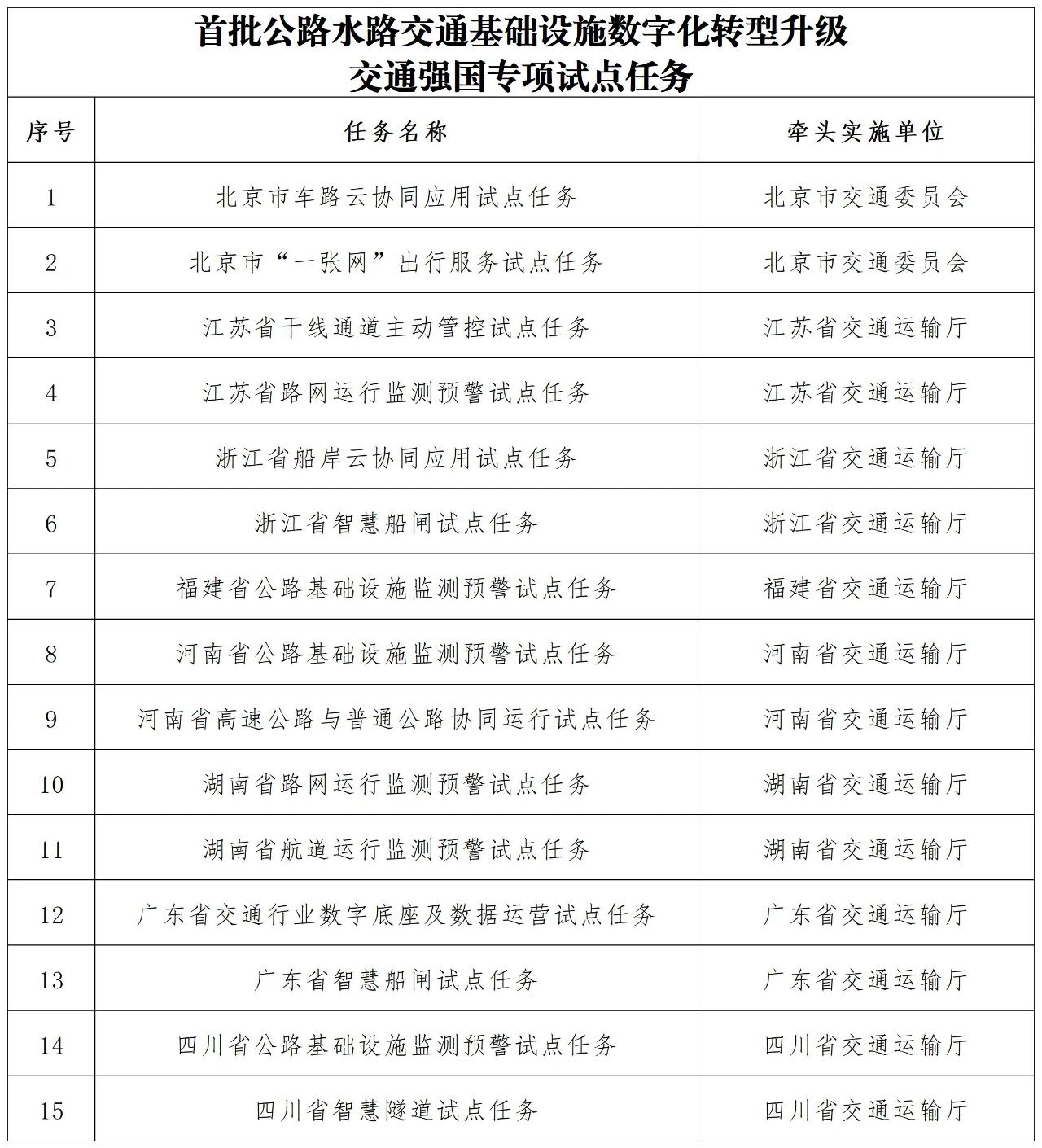
Xinhua News Agency reporter Guo Fangda
Just just after the Spring Festival holiday, the Tianjin Municipal Government issued the "Several Measures to Support the Innovation and Development of Biomedical in the Whole Chain in Tianjin", 25 policies are precisely drip irrigation from R&D to application, to help local governments explore and make good use of scientific and educational resources and clinical medical resources, continuously enhance biomedical innovation source and industrialization capabilities, and promote high-quality development of the biomedical industry.
The enrichment of innovative resources is a strong support for Tianjin's development of the biopharmaceutical industry. The local area has 31 national innovation platforms and 4 top 100 pharmaceutical companies in China. Relying on advantageous resources, Tianjin's biopharmaceutical industry has continued to make breakthroughs in the tracks of synthetic biology, gene therapy, high-end medical devices, etc., and accelerates its move toward a trillion-level industrial cluster.
Walking into the vaccine workshop of CanSino Biologics, the vaccines on the production line are undergoing orderly inspections. "Recently, we have developed an adsorption cell-free tetanus and disruption combined vaccine used in adolescents and adults." Zhu Tao, chief scientific officer of CanSino Biologics, said that with the support of policies, phase II and III clinical trial research will also be further accelerated. By the end of 2024, CanSino's project was selected as a major special project for biomedical science and technology in Tianjin.
Several measures propose to establish a "key research and development catalog for innovative products" and a "innovative product guidance application catalog" to open up a "highway" from laboratories to hospital beds for innovative drugs and devices.
The review time limit for supplementary drug applications is shortened to 60 working days, and the technical review of Class II medical devices is shortened to 40 working days. The role of Tianjin Medical Device Inspection and Testing Community has further compressed the time limit... The compression of review time provides convenience for the implementation of more achievements in the special tracks such as synthetic biology, gene therapy, and AI medicine.
A series of policy dividends are constantly giving rise to technological fission. Within the National Synthetic Biotechnology Innovation Center, carbon dioxide synthetic starch technology has exceeded kilogram mass production, and the "cell factory" on the assembly line is converting greenhouse gases into food raw materials.

In the laboratory of Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, researchers are discussing the progress of the work. Photo by Xinhua News Agency reporter Sun Fanyue
"In 2021, the institute achieved a breakthrough in the direction of carbon dioxide bioconversion, and achieved the de novo synthesis of carbon dioxide to starch for the first time in the laboratory. Now we have made progress in mass production." Xiang Hua, director of the Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, said.
The R&D cycle in the biomedicine field is long and the investment is high, and capital is the source of confidence for enterprises to deepen their cultivation. "When you are in the head, you can clearly see the monthly expenditure, and you will silently calculate in your mind how long it can last." Sun Jun, founder of Tianjin Zhonghe Gene Technology Co., Ltd., said that "finding money" has always been a difficult problem faced by technological innovation enterprises in the initial stage.
In early 2024, with the joint support of the competent authorities and the funding parties, Zhonghe Gene completed the delivery of the last Pre-A round of financing; in January 2025, in a synthetic biological manufacturing innovation and entrepreneurship competition, Zhonghe Gene was once again favored by many investors. "The enterprise has survived the 'darkness before dawn'," said Sun Jun.
Zhu Yubing, director of Tianjin Science and Technology Bureau, said that in the future, Tianjin will also strengthen financial support, give full play to the role of government-guided funds such as Angel Investment Guidance Fund and Haihe Industrial Fund, and actively attract social capital to jointly create a multi-level biomedical fund group with a total scale of over 10 billion yuan.
In 2024, the output value of industrial enterprises above the designated size in Tianjin exceeded 90 billion yuan, an increase of 6.96% year-on-year. The total investment of 188 projects under construction exceeded 53.5 billion yuan, and the industrial ecology showed a "rainforest-like" prosperity.
"We will continue to base ourselves on the actual development of the industry, consolidate traditional advantage areas, lay out emerging tracks such as cell and gene therapy, brain-computer interfaces, etc. in advance, strengthen the supporting role of innovative source-first-in-progress advantages such as synthetic biology on the industry, and continuously expand and strengthen the scale of Tianjin's biopharmaceutical industry." Zhu Yubing said.